Cytokine Panels
Contact OncoHelix to arrange for Cytokine Profile Testing at your center.
Click to view our Enhanced Cytokine Profile Report.
Click to view our Cytokine 2-Plex Report.
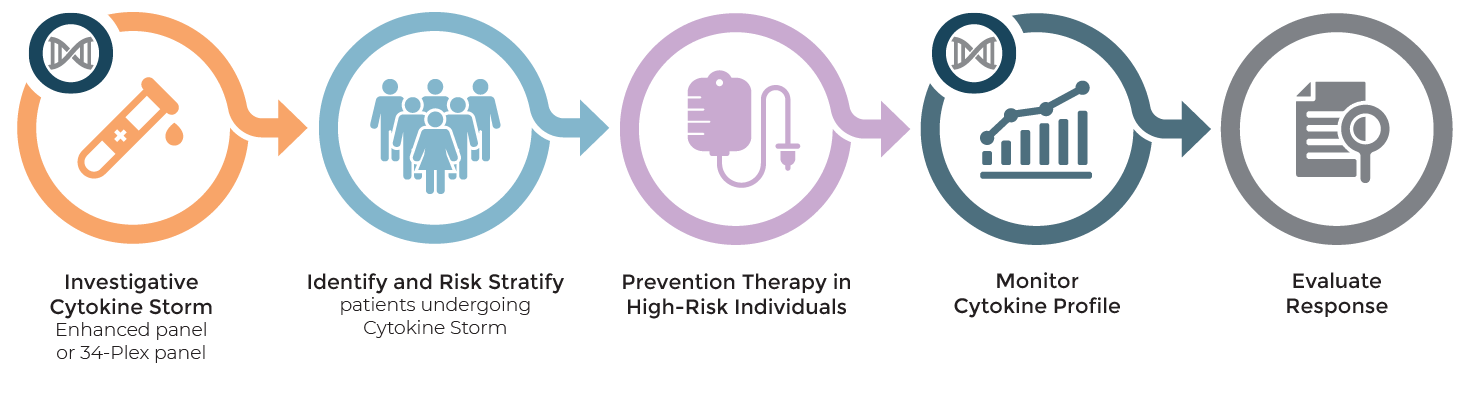
OncoHelix provides the ability to determine a person’s cytokine profile to investigate and risk-stratify those individuals undergoing cytokine storm/cytokine release syndrome. Traditional single cytokine tests and cytogenetic assessment is limited and non-specific in predicting those patients at high risk for cytokine release syndrome or cytokine storm.
INVESTIGATE and MONITOR the Storm with OncoHelix
At-risk patients for cytokine syndromes include:
- CAR-T treatment related oncology patients
- Acute inflammatory conditions like hemophagocytic lymphohistiocytosis (HLH)
- COVID-19 related patients (see COVID-19 Cytokine Storm tab for more information)
Cytokine Profile Difference
Using a Luminex based serum immunologic test, performed by clinical diagnostic facilities of OncoHelix, three panels – Enhanced, 34-plex and 2-plex cytokine and soluble receptor profiling panels - identify:
- Specific Cytokine profile compared to a baseline of 132 normal healthy people (unlike non-specific traditional cytokine tests)
- Changes in an individual’s cytokine profile: monitoring of disease progression and/or therapy response
- Investigation of an inflammatory disease
- Diagnosis of acute inflammatory conditions like Hemophagocytic Lymphohistocytosis (HLH)
- Investigation and monitoring of Cytokine Storm
Enhanced Cytokine & Soluble Receptor Profile:
34-PLEX Cytokine Panel
Analytes (pg/mL and reference ranges): Eotaxin/CCL11, GM-CSF, GRO alpha/CXCL1, IFN alpha, IFN gamma, IL-1 beta, IL-1 alpha, IL-1RA, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8/CXCL8, IL-9, IL-10, IL-12 p70, IL-13, IL-15, IL-17A, IL-18, IL-21, IL-22, IL-23, IL-27, IL-31, IP-10/CXCL10, MCP-1/CCL2, MIP-1 alpha/CCL3, MIP-1 beta/CCL4, RANTES/CCL5, SDF1 alpha/CXCL12, TNF alpha, TNF beta/LTA.
2-PLEX Soluble Receptor Panel
Analytes (pg/mL or ng/mL and reference ranges): sIL2Ra and sCD163
Anti-cytokine syndrome therapies
MONITOR for RESPONSE
OncoHelix can support cytokine profile panel testing to assess response to anti-cytokine (e.g., anti-IL-1, anti-IL-6 or anti-TNFa therapy) or other therapies with continued serum testing after treatment has been initiated.
Clinical Pathways
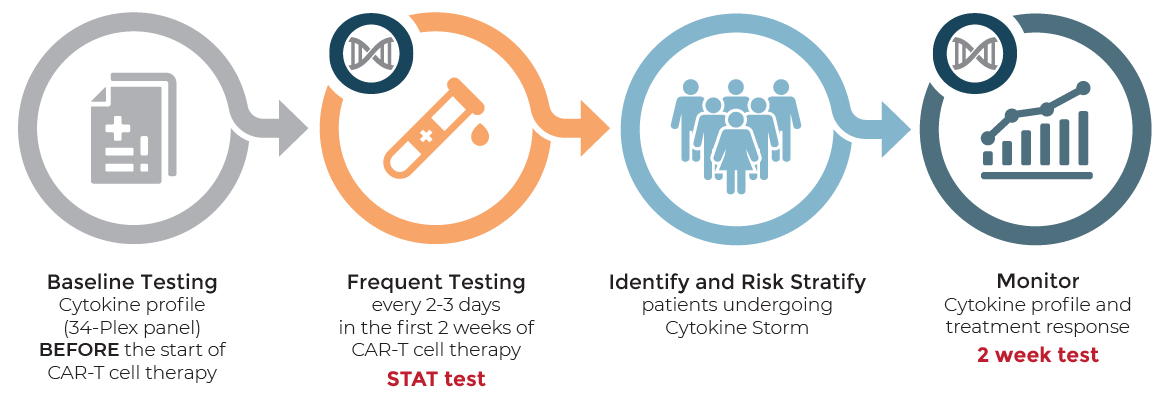
Cytokine Syndromes Testing and CAR-T Oncology Treatment
CLINICAL GRADE Cytokine Profiling Facility
Hematology Translational Lab (HTL), Calgary, AB
The HTL is a is clinically accredited, province-wide utilized, and a trusted lab at the Arnie Charbonneau Cancer Institute in the University of Calgary, providing clinical grade cytokine profiling. HTL is official testing facility for OncoHelix.
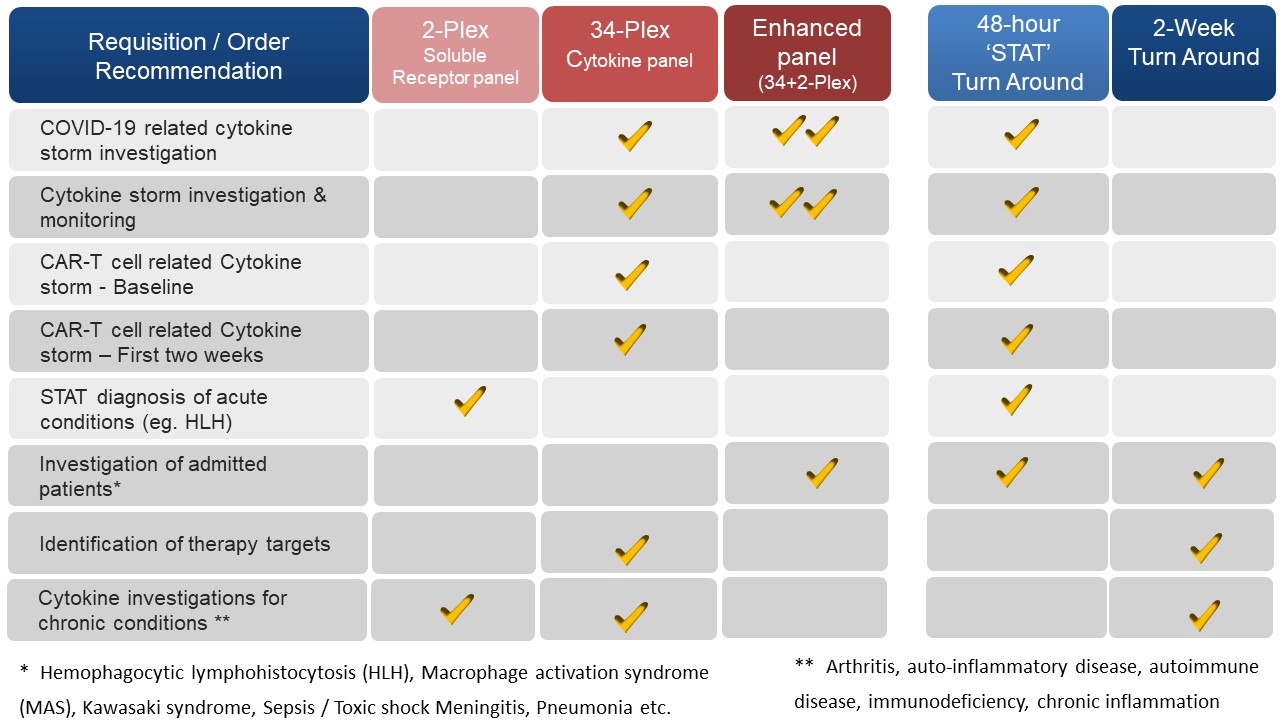
Turn Around Time:
- 2-week routine option
- 48 hour ‘STAT’ option
Recommendations for ordering Cytokine Profile Panel based on Turn Around Time
References:
Shimabukuro-Vornhagen et al. Cytokine release syndrome
https://doi.org/10.1186/s40425-018-0343-9
